Coronavirus is a type of virus that is known for the cause of serious respiratory illnesses including common cold leading to a fatal and contagious disease named coronavirus disease (COVID-19).
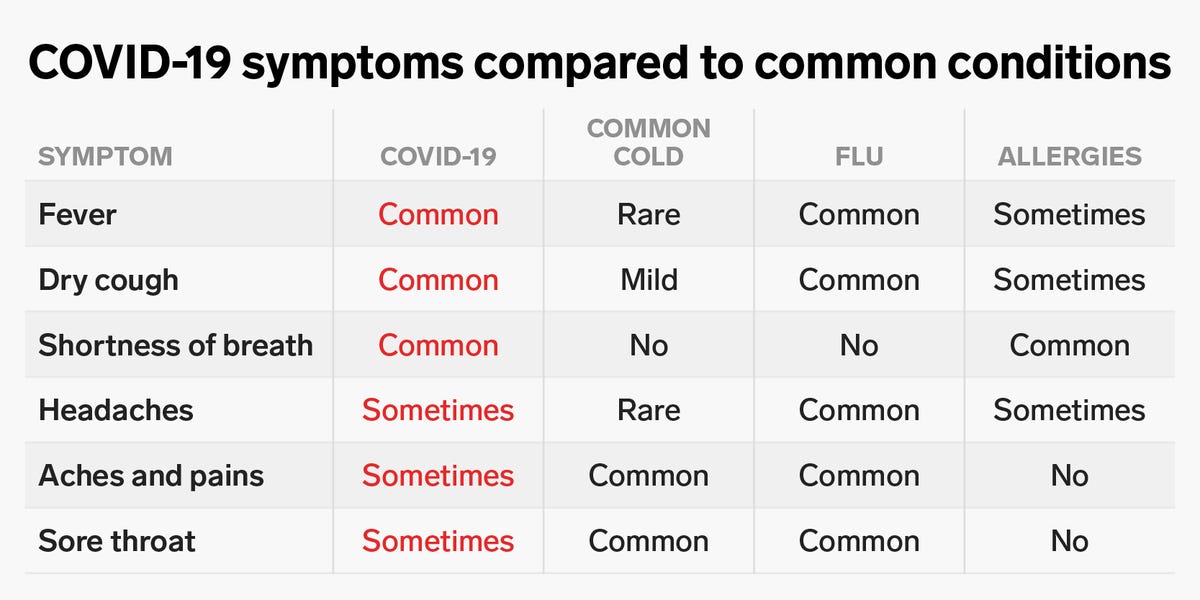
The most common symptoms for this disease may include; fever, runny nose, body aches and shortness of breath (in severe cases). These symptoms can be confused with the symptoms of some other common diseases such as; flu and the common cold, which makes it diagnosis more complicated. However, the development of diagnostic kits have been successfully achieved and proving to be highly useful in this situation but the availability of these kits is still a serious issue.
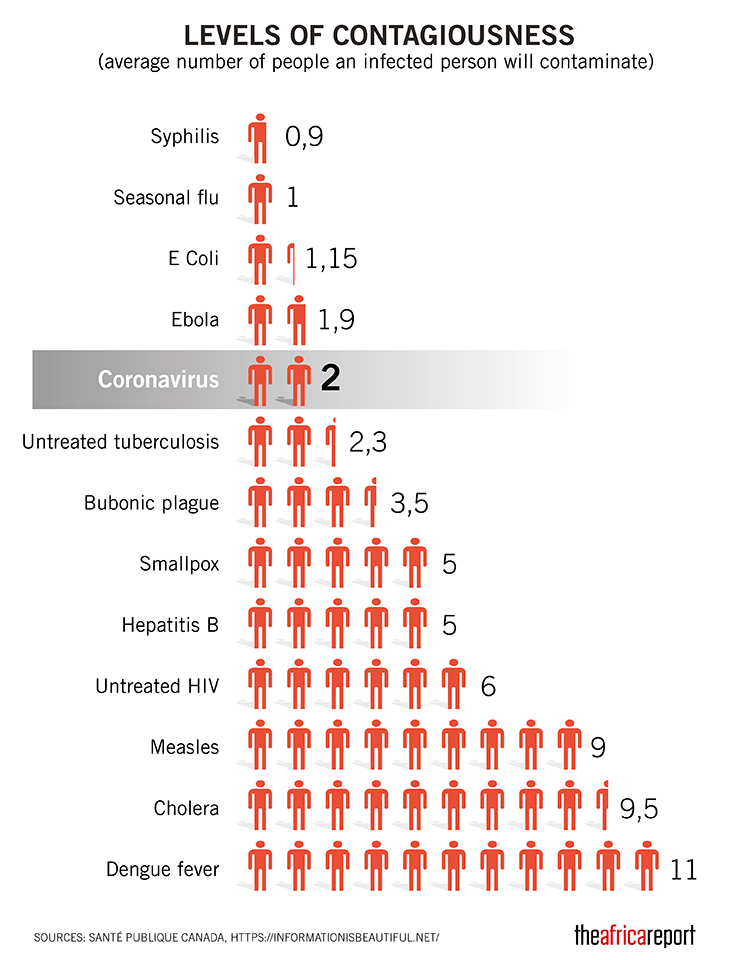
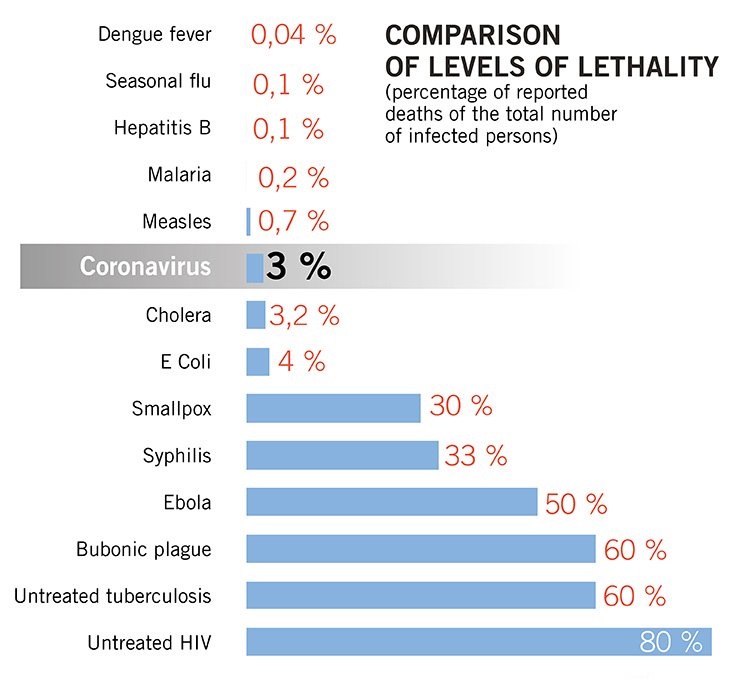
The major fear of this virus is its rapid spread as according to an initial WHO report, 01 infected person can transfer this virus to 2.6 persons which can accelerate the spread on a high level. Recent researches have evaluated that this virus is airborne as its stay time in the air is approximately 08 hours which makes the condition more critical.
At the end of 2019, an outbreak of this coronavirus took place in Wuhan, the city of China. The wide-spread of this virus in almost the whole world led to the occurrence of a pandemic. Not only China but almost the whole world has been hit really hard by this virus and it has proven itself fatal.
It has created fear and panic among the population of the whole world. Meanwhile, the whole healthcare profession including the researchers came under high pressure of this disease and the day-by-day worsening situation. But they are struggling to find the treatment for this fatal and contagious disease.
All the Pharmaceutical companies are running their experiments and trials to come up with a vaccine candidate which can help to save the world. They are focusing on the products which can be used to prevent the occurrence of this disease. Every researcher is trying to work on a novel perspective that can be useful to fight this novel virus. As some are working for the induction of antibodies in the human body while some others are working on the vaccine candidates which will also boost the response of the human immune system for these vaccines leading to a fast response.
Following are a number of vaccines by different research organizations and pharmaceutical companies which are expected to be useful to fight this novel COVID-19 and to help in flattening the curve. Some of these vaccines are already in the clinical trials while others are hoping the best results from their preclinical clinical trials which will lead them too to the clinical trial conductance.
Coronavirus (COVID-19) Vaccines in 2020
The following are vaccines that are under trial and expected to be available by the end of 2020.
-
CureVac Vaccine
It is an mRNA based vaccine which is expected to treat the pandemic coronavirus. The clinical trials have not been conducted yet to test the efficacy and safety of this vaccine in patients. But according to reports, it has been known that the first trial will be carried out in April in animals and the results of this trial will lead to further human-based clinical trials in early summers of 2020. In the market, a few rumors have also been heard which states that the USA tried to lure the development of this vaccine to the country from Germany to restrict its production for its public only. But at many platforms, these kinds of statements are denied by the concerned people.
-
Ii-Key peptide vaccine by Generex Biotechnology
This immune system enhancing vaccine basically consists of a peptide. Li protein has a portion or segment which is called a li-key peptide which is observed to have the greater potency for the mechanism of receptor binding to enhance the overall function of the vaccine.
It is observed that the amino acid-based sequences of SAR-CoV-2 have a greater vulnerability for binding to these li-key peptides. It is aimed by the Generex to choose the best li-key peptides and develop a vaccine on an urgent basis leading to human clinical trials. It is planned that the development of this vaccine will be carried out in the USA and funded by China.
-
S-Trimer by GlaxoSmithKline (GSK) and Clover
This novel COVID-19 has to lead the GSK to make a partnership with Clover to produce this protein-based vaccine on an urgent basis. It has been stated by GSK that Clover has the best and most commercial in-house cGMP followed bio-manufacturing facilities in China which can accelerate the development of this particular vaccine in these pandemic conditions. GSK has given the approval to Clover to run the preclinical trials with this protein-based vaccine for the further evaluation of safety and efficacy. As well as to determine the potential of this vaccine to restraint this pandemic situation in the world.
-
Johnson & Johnson (J&J) + Beth Israel (BIDMC)
Previously, Johnson and Johnson developed the vaccines for Zika and HIV which tremendously helped the public to deal with these diseases. These two foundational developments were done by the collaboration of BIDMC and taking it into account, J&J decided to work with them again to bring another innovation to deal with the situation of pandemic coronavirus. The development of a preventive vaccine for the novel COVID-19 has been planned by the concerned authorities. It has been stated that the preclinical trials are already in run by BIDMC and the results making us expect the start of a clinical trial of this evaluated vaccine by the end of March 2020. It has also been added to the statement that J&J is working to expand its in-house bio-manufacturing capacities to accelerate the development of this vaccine to meet the global needs of vaccines in this pandemic situation.
-
Linear DNA vaccine by LineaRx
Polymerase Chain Reaction (PCR) has been used to make the linear DNA vaccine against the novel COVID-19. It has been stated that four vaccines will be developed which will be evaluated further leading to the best choice against coronavirus. These vaccines are developed on the design of the “spike” protein which is considered to be responsible for the uptake of coronavirus for enabling the effective binding of receptor sites on the host cells. This way, these vaccines are expected to be useful to mimic the action of this spike protein against this virus. After the animal-based preclinical trials, these DNA based vaccines are expected to be shipped for further evaluation and clinical trials.
-
Vaccine and antibody by Medicago
It is expected that Medicago is in action for the development of Virus-Like Particle (VLP) vaccines and antibodies to fight against SAR-CoV-2. This pandemic has lead Medicago to make a partnership with the inventors of the Ebola vaccine to accelerate the development of these VLP vaccines and antibodies. It has been stated that by using a plant-based technology platform, the first step of vaccine production has been successfully completed. This step involves the development of a novel coronavirus VLP which has been produced just after the 20 days of receiving of SAR-CoV-2 sequences. The clinical trials are expected to be carried out in July and August of this year, 2020.
-
Oral Recombinant Vaccine by Vaxart and Emergent Bio
The development of an oral recombinant vaccine in the tablet dosage form has been expected from the recently made partnership of Vaxart and Emergent under “molecule to market” contract services. It has been stated that Emergent will be responsible for the development and manufacturing of these experimental oral vaccine candidates. Meanwhile, the Phase 1 clinical trial study is expected to be carried out in the last half of this year, 2020 by Vaxart to further evaluate the efficacy and safety of these candidates. Emergent has claimed to provide their best services for this development to meet the recent pandemic challenges.
-
S-Spike by CSL and The University of Queensland (UQ)
It is reported that the University of Queensland was working to develop the viral protein with its molecular clamp technology and taking this into consideration, CSL approached them and offered their MF59 adjuvant to carry out further studies. This MF59 adjuvant is expected to be used in the preclinical trials by the UQ to test the potential of vaccine candidates against novel coronavirus. After 09 days of this partnership, UQ claimed that the molecular clamp technology can be useful for the development of vaccines against nCOVID-19 which will be readily recognized by the immune system leading to the accelerated production of natural antibodies in the system to fight against this novel virus.
-
BNT162 by Pfizer and BioNTech
BNT162 is potentially considered to be in the first class mRNA based vaccines which are expected to be designed for the prevention of novel COVID-19 infections by inducing the immune system of the human body.Pfizer and BioNTech made the contract for the development of this vaccine and named this project as “Project Lightspeed”. They claimed to start the clinical trials for BNT162 in April of 2020 but all the information regarding the development, production, and marketing has not been disclosed yet by both of these companies. Meanwhile, it has been stated that Focus Pharma will also be included in this project to accelerate the production of this vaccine in China to fight this novel coronavirus.
-
Moderna Therapeutics Vaccine
mRNA-1273 is a novel vaccine encapsulated in lipid nanoparticles that have been developed by Moderna to encode the Spike protein’s perfusion stabilized form to fight against the pandemic nCOVID-19. On March 16th the first clinical trial of this novel vaccine was started in the USA to check the safety, efficacy, and toxicity of this candidate. This clinical trial is based on the dose-ranging and open-label use of the mRNA-1273 vaccine so that the transparent results regarding the safety and efficacy if this candidate can be achieved. In this trial, the healthy subjects are administered with the three dosages of the vaccines, 28 days apart following the two-dose vaccination schedule.
 Health & Care Information
Health & Care Information