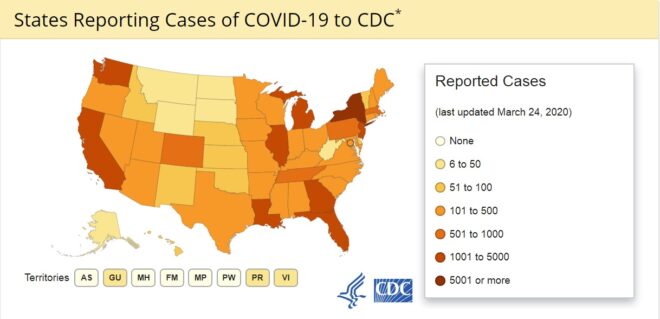
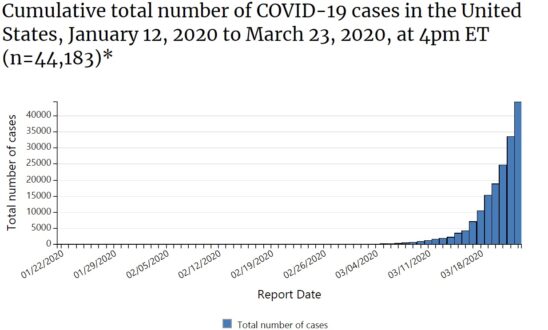
Coronavirus is a type of virus that is known for the cause of serious respiratory illnesses including common cold leading to a fatal and contagious disease named coronavirus disease (COVID-19).
This deadly virus was originated from Wuhan, the city of China at the end of 2019 which has infected thousands of people so far, around the globe. Life of millions of people worldwide has been disrupted by this novel virus. The Chinese have somehow overcome this situation but the condition in other cities especially Italy and Iran is very critical right now. This situation is desperately in the need of a treatment that can help to fight it.
Initially, when this virus originated and started to spread no treatment was available at that time which leads to panic situations in the public. Not only the public but the whole healthcare profession including the researchers’ society came under a lot of pressure to come up with some new treatment. The panicked and ill-informed public also fell for some scams such as essential oils and nano-silver treatments. However, it has been stated by the Food and Drug Administration (FDA) that there is no approved treatment, cure or prevention for this novel coronavirus, so far.
The initial cases of novel coronavirus were given the symptomatic treatment to reduce the severity of the symptoms leading to the relief of the patients. Many patients were recovered with this symptomatic treatment but the death toll in China and Italy went to the highest. However, the researchers are busy in finding the drug agents which can help to reduce the symptoms of the disease leading to the survival of the patients.
As it is a fact that a new innovation takes almost 15-20 years to reach the market following the normal cycle of drug development. Even the quickest development cycle cannot be expected to complete in a year which is the need of this pandemic 2020. Keeping this fact in view, a very sensible statement was made by Pfizer pharmaceuticals as they said that they are examining their pipeline antiviral drugs and evaluating those under-developed drugs which can be useful against SRAS-CoV-2 strains. As the desperate need of treatment is posed by this pandemic which left us with the only solution to repurpose the already existing antiviral drugs and check their efficacy and safety along toxicity to provide the immediate solution to this widely spreading contagious disease.
Coronavirus (COVID-19) Medicines in 2020
However, the efforts of the researchers and pharmaceuticals are also appreciable who are working for the upbringing of innovations to deal with this novel virus. But until the absence of any approved drug to fight the nCOVID_19, repurposing sounds like a wise choice in this pandemic condition.
-
Kaletra by AbbVie
Kaletra is an HIV medicine comprising of a combination of two antiviral drugs including; lopinavir and ritonavir, and it is commonly called as Aluvia. When the novel COVID-19 emerged abruptly in Wuhan, the City of China, this drug was considered important as a treatment option. A clinical trial was carried out from 18th January to 3rd February, including 199 infected patients who were administered with this HIV medicine to check the efficacy. The results came out from this trial were disappointing as no signs of improvement of symptoms, reduction in the number of pathogens or shortage in days of hospital stay were observed. But there is more to the story, as it has been brought to the light that the enrollment of the patients in the study was not accurate. All the patient were at their critical stages of the viral infection which drastically affected the results. So, this drug cannot be considered as a dead-end yet.
-
Ganovo by Ascletis Pharma
Danaoprevir is a drug marketed under the brand name Ganovo by Ascletic Pharma. After the recent outbreak of nCOVID-19, healthcare professionals came out with a treatment option which includes the combination of Ganovo with Ritonavir. A small sampled clinical trial was run to check the effectiveness of this combination which included only 11 patients. It has been stated that the results of this clinical trial were quite promising as all the patients from this study have been discharged after satisfying the Discharge Standards issued by China’s National Health Commission, under “Diagnosis and Treatment Program for Novel Coronavirus Infection (Trial Version 6)”.
-
Chloroquine (CQ) or Hydroxychloroquine (HCQ)
These two anti-malarial drugs are officially considered to be very effective for the treatment of novel COVID-19. Numerous clinical trials have been carried out to investigate the safety and efficacy of these drugs in the nCOVID-19 patients. The outcomes of these trials were quite promising as improvement in CT images and reduction of symptoms have been observed in the subjects under study. In some studies, it has been mentioned that Hydroxychloroquine (HCQ) is considered to be safer than Chloroquine (CQ) as CQ poses some serious side effects. On the other hand, HCQ is cheaper and less toxic which makes it a better treatment choice for the nCOVID-19.
-
Galidesivir by Biocryst
It is a wide spectrum antiviral drug that has been proved to be effected against a number of rare viruses including; Ebola, Zika, Marburg and yellow fever. It has been reported that this ant-viral drug may also have potential effectiveness against the novel COVID-19. Clinical trials are carrying out to assess the efficacy of this drug against nCOVID-19 but it is still a way off from becoming an approved drug that could be used to combat this rapidly spreading serious viral concerns.
-
Anti-SAR-CoV-2 by Eli-Lilly and AbCellera
These are the antibodies that have shown the potential to prevent and treat the novel COVID-19. According to reports, these antibodies have been found in the blood of the first treated patient of coronavirus in the USA. After this fact, Eli Lilly and AbCellera have been agreed to make a partnership on the co-development of 500+ these completely human rare antibodies sequences. Firstly, the National Institute of Allergy and Infectious Diseases (NIAID) will evaluate the best match of antibodies which will potentially bind with the pandemic strains of the SAR-CoV-2. As a part of this theory, after this evaluation, these antibodies will be manufactured and their provision will be made easy to the patients.
-
Remdesivir by Gilead Sciences
It is an antiviral drug that was considered to be ineffective but now it has been studied to assess its effectiveness against the novel COVID-19. Several clinical trials are being carried out in different countries to investigate its safety and efficacy against newly emerged coronavirus. Firstly this drug was administered to a patient in the USA and the results were positive as it treated the patient. For further investigation, a clinical trial based on 761 patients is being carried out in Wuhan, the city of China and the results are expected in the coming few weeks.
-
Prezcobix by Janssen Pharmaceutical (Johnson & Johnson)
It is a combination of two drugs including; darunavir and cobicistat which are basically HIV protease inhibitors and might have the potential to work against the novel coronavirus. This is a type of vaccine which is going to be developed by the cooperation of two organizations in order to find the solutions of efficacy against the pandemic strains of SAR-CoV-2. Until 29 January, a total of 800 boxes of Prezcobix were shipped to two different research centers of China to carry out the clinical trials to evaluate the efficacy of this drug against this widely spreading novel coronavirus.
-
Antibodies SARS-CoV-2 by Johns Hopkins
A rare sequence of antibodies has been observed in the blood of the coronavirus patients that have been treated and recovered from the disease. It has been assumed that these antibodies are developed in the human body as a response of the body’s own immune system which fought against the SAR-CoV-2. Now just like a few other companies, John Hopkins is trying to do its best to adopt a different technique to accelerate manufacturing leading to the provision of these completely rare human antibodies to the patients. But they have also given the statement that this treatment option can only be utilized when there is enough number of recovered patients who can donate their serum containing these rare antibodies.
-
Actemra by Roche (Genentech)
This drug is considered very important as it was recommended to be used among the severe cases of coronavirus. In these cases, the main problem was organ failure followed by the overreaction of the immune system of the body as well the outburst of the cytokines, which can be controlled and treated by the use of this drug. Due to this potential, it has been decided by the concerned authorities that a clinical trial involving 188 coronavirus patients will be conducted in China until May 2020 to assess the efficacy of this drug. The outcomes of this clinical trial are expected to be presented by the end of 2020.
-
Arbidol by Pharmstandard
Umifenovir is a membrane merging inhibitor drug marketed under brand name Arbidol and it was basically indicated for the treatment of influenza. Overall six clinical trials are expected to be conducted to investigate the efficacy of this drug as a monotherapeutic product a swell in combination with other products (AbbVie’s Kaletra, Ascletis Pharma’s ASC09, lopinavir, ritonavir, carrimycin, and Bromhexine Hydrochloride) against the pandemic strains of the SAR-CoV-2. This drug will be accounted for among the Western medicines and the outcomes of these kinds of trials will be compared with the Chinese traditional medicines for the final evaluation of the efficacy of the monotherapy of this drug.
 Health & Care Information
Health & Care Information