Secukinumab (Cosentyx) cost, dosing and side effects as new FDA approved medicine for psoriatic arthritis treatment by East Hanover, New Jersey-based Novartis Pharmaceuticals Corporation.
Cosentyx also known as secukinumab is a new medicine was approved by US Food and Drug Administration (FDA) and the European Commission in January 2015. Inactive ingredients present in this product are trehalose dihydrate, L-histidine/histidine hydrochloride monohydrate, L-methionine, polysorbate 80, and water for injection.
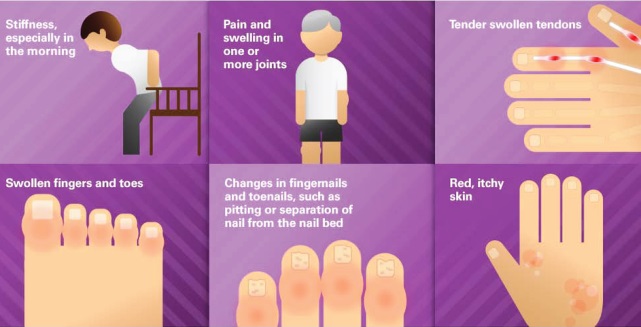
Psoriasis is a skin condition that causes irritation and makes red patches on the skin. Psoriasis is an autoimmune disease, and happens all the more ordinarily in patients in with a family history of the infection, and frequently starts in individuals between the ages of 15 and 35. The most well-known type of psoriasis is plaque psoriasis, in which patients grow thick, red skin with flaky, silver-white patches known as scales. Secukinumab the is an immunosuppressant that diminishes the impacts of a chemical substance in the body that can bring about inflammation. Consentyx is used to treat moderate to extreme plaque psoriasis (raised, shiny chipping of the skin) mostly in adults.
Secukinumab is a neutralizer that ties to a protein (interleukin IL- 17A) which is included in irritation. By trying to IL-17A, secukinumab keeps it from binding to its receptor and represses its capacity to trigger the provocative reaction that assumes a part in the improvement of plaque psoriasis.
COSENTYX® is a registered trademark of Novartis AG. All trademarks, service marks, logos, product label and packing images displayed on this website, are registered to the respective owner. Their use or mentioning on this website is only for informational purposes. Please ask your doctor or pharmacist for more information.
Cosentyx is managed as a subcutaneous injection i.e. infusion under the skin. It is proposed for patients who are the contenders for systemic therapy which is a treatment utilizing substances that go through the circulatory system, after being taken orally or infused), phototherapy (bright light treatment) or a combination of both.
Secukinumab (Cosentyx) Cost, Dosing, Side effects
Please find below the detail on Cosentyx cost, side effects and dosage for psoriasis treatment:
Secukinumab Side effects
All medicines may bring about reactions. However, numerous individuals have no, or minor, symptoms. Check with your physician if any of these most Common Cosentyx (Secukinumab) Side effects hold on or get to be irksome:
- Symptoms of cold
- Diarrhea
- Nose or throat itching
- Upper Respiratory Infections Symptoms i.e a mild cough, mild sore throat, runny/stuffy nose, sniffling
Look for medical attention immediately if any of these Severe Cosentyx Side effects happen:
Serious Hypersensitivity Responses
- Rash
- Hives
- Tingling;
- Trouble breathing
- Feeling faint
- Snugness in the mid-section or throat
- Swelling of the mouth, face, lips, throat, or tongue
Symptoms of Infection
- Fever, sweats, chills, or a tireless sore throat
- Cough
- Muscle throbs and aches
- Shortness of breath
- Warm, red, or excruciating skin
- Bruises on body
- Increased or painful urination
- Stomachache
- Blood in phlegm
- Unexplained weight reduction
Different medications may interact with secukinumab, including prescriptions and OTC meds (over-the-counter medicines), vitamins, and herbal medicines. Tell each of your healthcare providers about all medicines you use now and any prescription you begin or quit taking.
Secukinumab (Cosentyx) Cost for Psoriasis treatment
A potential price war among Novartis, Amgen and Eli Lilly & Co is expected over psoriasis drugs, as pharmacy-benefits managers pitch drug-makers against one another to win big discounts on the drugs.
There are 7.5 million psoriasis patients in the US alone. Psoriasis medicines can keep running into a large number of dollars. A tube of one of the new topical psoriasis drugs may cost in the middle of $500 and $600. The normal wholesale cost for each 150 mg prefilled syringe or pen is $2052. A generally new class of treatment for psoriasis and psoriatic joint arthritis can run $15,000 to $20,000 a year.
Secukinumab (Cosentyx) is considered a specialty medication, which means it is expensive. The current standard treatment medication for Psoriasis, Stelara costs $7,661 per injection for patients weighing under 220 pounds. It is manufactured by by by Johnson & Johnson. Novartis decided Cosentyx cost same as Stelara. Secukinumab cost in the US is $14,881 for 4 cartons (2 sensor ready pens). This is the Cosentyx 150mg/ml price.
Bloomberg draws a Cosentyx vs Ixekizumab vs Brodalumab price comparison and forecasts the sales of these drugs by 2019 as:
- Novartis’ Cosentyx $1.06 billion
- Eli Lilly’s Ixekizumab $558 million
- Amgen & AstraZeneca’s Brodalumab $432 million
On the other hand, in recent years, rising medication expenses and insurance troubles have consolidated to make biologics and different psoriasis medicines excessively costly for a developing number of individuals. Since 2006, the National Psoriasis Foundation, the main support association for psoriasis patients, has seen just about a four-fold increment in the quantity of individuals looking for help paying for psoriasis medicine.
Secukinumab (Cosentyx) Dosing for Psoriasis Arthritis Treatment
Cosentyx is to be directed by subcutaneous infusion. On the off chance that possible regions of the skin that show psoriasis must be avoided as injection sites
- Secukinumab Injection: Each pre-filled syringe contains 150 mg secukinumab in 1 ml.
- Secukinumab Injection: Each pre-filled pen contains 150 mg secukinumab in 1 ml.
- Secukinumab Injection: 150 mg, lyophilized powder in a single-use vial for reconstitution only for use of professional health care providers
The prescribed dose is 300 mg of secukinumab by subcutaneous infusion with beginning dosing at Weeks 0, 1, 2 and 3, trailed by month to month support dosing beginning in Week 4. Each 300 mg dose is given as two subcutaneous infusions of 150 mg.
Consideration must be given to discontinuing treatment in patients who have presented no response up to 16 weeks of treatment. A few patients with partial reaction initially might subsequently improve with continued treatment beyond 16 weeks.
Serum levels of aggregate IL-17A (free and secukinumab-bound IL-17A) are at first expanded in patients getting secukinumab. This is trailed by a moderate decline because of diminished leeway of secukinumab-bound IL-17A, demonstrating that secukinumab specifically captures free IL-17A, which assumes a key part in the pathogenesis of plaque psoriasis.
Indicated for moderate-to-extreme plaque psoriasis in patients who are receiving systemic treatment or phototherapy
- Introductory: 300 mg SC at weeks 0, 1, 2, 3, and 4
- Monthly maintenance: Beginning at week 8, give 300 mg SC once a month
- For a few patients, a dose of 150 mg may be acceptable
Secukinumab (Cosentyx) Dose for Kids
The safety and viability of Cosentyx in children below the age of 18 years have not yet been built up. Therefore, no information is available on Cosentyx (Secukinumab) dosage for Kids.
 Health & Care Information
Health & Care Information 



I am also a patient of plaque psoriasis plz tell me the complete and sure treatment of this disease